Pfizer vaccine results published in peer-reviewed journal
Stay tuned with 24 News HD Android App

The full results of a clinical trial for the Pfizer-BioNTech Covid-19 vaccine were published in the New England Journal of Medicine on Thursday, a major milestone that came as a committee of the US Food and Drug Administration met to discuss its approval.
An editorial related to the scientific paper said: "The trial results are impressive enough to hold up in any conceivable analysis. This is a triumph."
The full trial included almost 44,000 volunteers, several thousand more than the number seen in prior analyses. Around half received the vaccine and the rest a placebo.
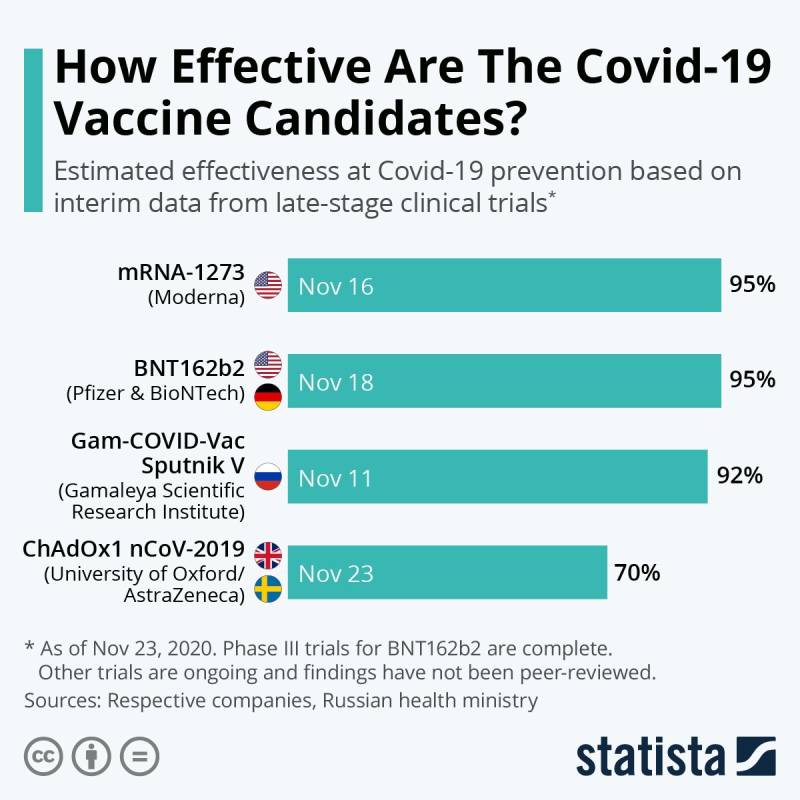
The paper confirmed that a two-dose regimen of BNT162b2 was 95 percent effective in preventing Covid-19 infection.
The vaccine worked similarly across "age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions," the paper said.
Among 10 cases of severe Covid-19 after the first dose, nine occurred in placebo recipients and one in a person who received the vaccine.
The editorial that accompanied the study did flag certain "minor issues."
"The number of severe cases of Covid-19 (one in the vaccine group and nine in the placebo group) is too small to draw any conclusions about whether the rare cases that occur in vaccinated persons are actually more severe," it said.
Other questions include whether unexpected safety issues may arise when the number of people vaccinated grows to millions and possibly billions of people.
Also unknown is whether more side effects will emerge with longer follow-up, how long the vaccine remains effective, whether it will limit transmission, and how it will work in children, pregnant women, and immunocompromised patients.
