DRAP officials raid offices of Avastin distributors in Lahore
Stay tuned with 24 News HD Android App

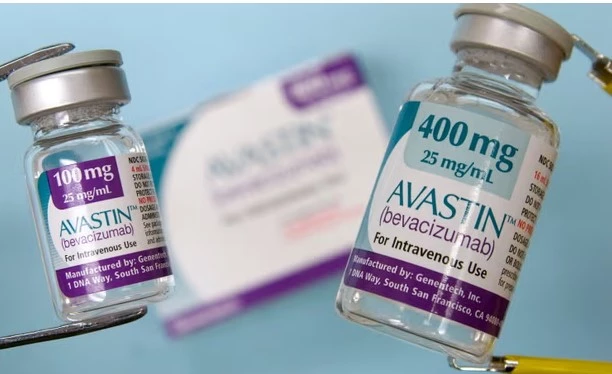
The officials of the Drug Regulatory Authority of Pakistan (DRAP) have raided the offices of the distributors of locally made adulterated Avastin injection along with provincial drug inspectors in Lahore, reported 24NewsHD TV channel on Monday.
Sources revealed that the DRAP officials raided the local distributors of injection manufacturer Rawish Pharma – AG & Company – and recovered 110 vials of Avastin injection.
The officials sent the recovered samples of the injection to the Drug Testing Lab in Lahore.
DRAP has stopped the distributors from selling the controversial injection.
The DTL will examine the quality of the injection and run a safety check on it.
It has been learned that Genius Advanced Pharma is selling injections of the same batch illegally.
Earlier, the Punjab health authorities registered a case against the manufacturers of the spurious eye drug that led to the vision loss among several diabetic patients, including the brother of senior PPP leader Chaudhry Manzoor Ahmed.
Deputy Drug Controller Hafiz Alam Sher of the chief drug controller office of Punjab lodged an FIR against Naveed Abdulllah of Kahna and Bilal Rasheed, alleging that they manufactured the ‘controversial drug’ on the premises of a private hospital, Saira Memorial Hospital in Faisal Town Lahore, and supplied the same across the province.
Avastin is a drug used to treat wet age-related macular degeneration (AMD).
It is also used to treat diabetic eye disease and other problems of the retina. It is injected into the eye to help slow vision loss from these diseases. Avastin is the brand name for the drug, which is called bevacizumab.
Several diabetic patients in Lahore, Kasur and Jhang districts had been administered Avastin injections to address retinal damage, but these injections led to severe infections, ultimately resulting in the loss of eyesight of around 12 patients.
The Punjab government on Sunday banned the sale and usage of the injection, allegedly responsible for causing loss of vision in several patients in the province for two weeks until quality check results poured in.
On Saturday, the Punjab government also formed a five-member committee of experts to look into the matter and report in three days.
https://twitter.com/MohsinnaqviC42/status/1705983541597688117
Reporter Kifayat Ali Shah
